Matt Hancock appears emotional over coronavirus vaccine rollout
The drug, which has been made in conjunction with AstraZeneca was found to be 90 percent effective once administered with half a dose and then a full dose.
In the data released today, the data also showed there had been no hospitalisations to those who received the drug in trials.
Oxford University and AstraZeneca have also confirmed the date released in the Lancet journal today, has now been passed to the regulators for approval.
Once approved, the UK could have two highly efficient vaccines following the rollout of Pfizer and BioNtech’s drug today.
Health Secretary, Matt Hancock claimed he is hopeful the authorities will grant approval in the coming weeks.
He said today: “We very much hope that it will get approval.
“We hope that to come through in the next couple of weeks.

Coronavirus: Oxford’s vaccine found to be 90 percent effective (Image: GETTY)
Coronavirus vaccine: The drug could be weeks away (Image: GETTY)
“It has to be a decision for the independent MHRA, who will only approve it if it is safe to use and effective. So that works underway and I wouldn’t expect anything for the next couple of weeks.”
The data was based on three trials in the UK and Brazil which included 11,636 people.
Trials did also find the drug had 62 percent efficacy when administered with two full doses.
Amid the differing of the results from two different methods of administering the drug, the overall efficiency was found to be at 70.4 percent.
JUST IN: Michael Schumacher health update: Mick caught out

Coronavirus vaccine: The drug was found to be 90 percent effective (Image: GETTY)
Sarah Gilbert, professor vaccinology at the University of Oxford said today: “The really good news is that from three weeks after the first dose of the vaccine – and that’s the first dose, not the second dose – there were no hospitalisations or severe cases of Covid in the participants in this trial and that is the kind of effect that will have a really huge impact on health systems.
“And then the next level of protection that we’re looking at is against symptomatic PCR confirmed infection. And that’s the main readout that we report today.
“We are getting weekly swabs from people and we are seeing an effect on reducing the asymptomatic infections as well.
“So overall: protecting against severe disease and hospitalisation; strong impact on symptomatic disease and some impact on asymptomatic infection.
DON’T MISS
Matt Hancock ‘confident’ Britons will resume holidays in 2021 [Latest]
Covid vaccine hospital hubs: Where are the vaccine hubs? [Update]
EU shatters unity– tells Poland and Hungary you can’t stop us [Insight]

Coronavirus: Matt Hancock expressed his hope for the drug to be rolled out in weeks (Image: PA)
Coronavirus: The drug is administered with two doses (Image: GETTY)
“So that’s going to have quite a large effect on transmission.”
Further research will be conducted into the long-term immunity of the drug going forward.
The drug is seen as incredibly important due to its price in comparison to Pfizer’s.
Unlike Pfizer’s drug, the vaccine does not need to be kept at ultracold temperatures.
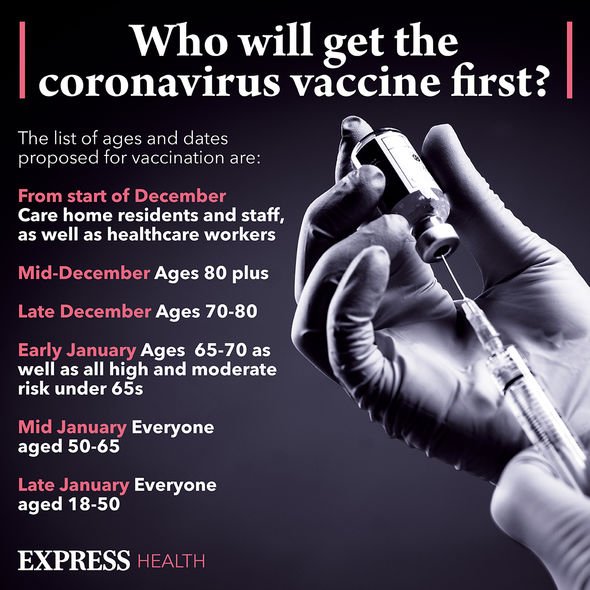
Vaccine priority (Image: Express)
The drug costs £3 which is far less than Pfizer’s £15 and Moderna’s £25 vaccines.
The Government has signed a deal to acquire 100 million doses of the drug.
AstraZeneca has also stated its intent to manufacture three billion doses of the vaccine throughout 2021.
Vaccinations began today (Image: PA)
Professor Andrew Pollard, director of the Oxford University Vaccine Group said today: “I think what we need are vaccines in people’s arms.
“I think there’s a really important point that the only way that we get the pandemic behind us is to have doses of vaccines out there in populations here and around the world.
“And personally I think we need to be completely agnostic about which vaccine is being used because we need multiple vaccines, as soon as possible.
“Otherwise we’ll still be in this position in six months’ time. Whereas now we are starting to get to a point with three vaccines showing efficacy of starting to get the pandemic behind us.”







