Let’s Talk … How long plasma treatment lasts?
PoliticMag Press Release : November 20, 2020
The most commonly asked question concerning plasma surface treatment is; How long does plasma treatment last?
In this ‘Let’s talk …’ article we discuss the main factors that influence the lifetime of plasma surface treatments and explain how these factors can affect different materials.
Plasma surface treatment is an established and effective method of improving the adhesion characteristics of a wide range of materials. Even for the most challenging polymers such as PET, PPS, PEEK, PTFE, acetals (POM), polyamides and polyolefins, plasma treatment is a fast and reliable process to increase the surface energy of a material, thereby making it easily wettable.
Wettability refers to the ability of ink, paint or adhesive to spread on a surface and form intimate and strong chemical bonds with surface molecules. Most polymers are naturally hydrophobic, meaning that liquids bead up on the surface and do not form the large numbers of strong bonds which are a pre-requisite for good adhesion. Metals, glass and ceramics by contrast are naturally hydrophilic, however surface contamination is often present from the machining/production processes which impede adhesion to these materials also. Surface contamination may also be present on many engineering polymers in the form of e.g. mould release agents, plasticizers, pigments and antioxidants.

Plasma surface treatment works by a two-fold action; (i) removal of contamination from the surface (cleaning) and (ii) increasing the surface energy by the attachment of oxygen-containing molecules (activation). Cleaning takes place on all surfaces whereas activation tends to apply mainly to polymers.
What causes Plasma Treatments to decay?
In the case of plasma cleaning, parts will tend to remain in a cleaned state indefinitely as long as they are stored under clean conditions and are free from atmospheric contaminants or from the transfer of oils and salts by e.g. touching with bare skin.
The situation is a little more complex in the case of polymers. The cleanliness factors above still apply but surface activation is now dominant in increasing the surface energy of the material. The reduction in the effectiveness of plasma treatment over time, i.e. the reduction in surface energy, is known technically as hydrophobic recovery. The bulk properties of a plasma-treated material remain unchanged in comparison to the surface and over time the molecules from within the bulk may migrate to the surface through thermodynamically driven reorientation, causing the oxygen-containing polar groups to be relocated underneath the surface.
This recovery is to a large extent a characteristic of the material rather than the plasma treatment and we will discuss some reasons for this below.
Type of material
The characteristics of the material undergoing plasma treatment are one of the most important factors that affect the rate of hydrophobic recovery. Some polymers are more tightly packed, and the sub-surface molecules have less freedom of movement, hindering their ability to relocate to the surface and thus slowing down the recovery process. Conversely, lower density polymers can more easily reorientate and the oxygen-containing surface molecules will in turn migrate into the bulk. Low molecular weight additives such as plasticizers and pigments will, if present, also migrate back to the surface over time.
Figure 1 demonstrates this difference by comparing the hydrophobic recovery of low-density polyethene (LDPE) and high-density polyethylene (HDPE). LDPE recovers to a lower energy state (higher water angle) than HDPE, although both materials retain a higher surface energy than the untreated materials, even after several weeks.
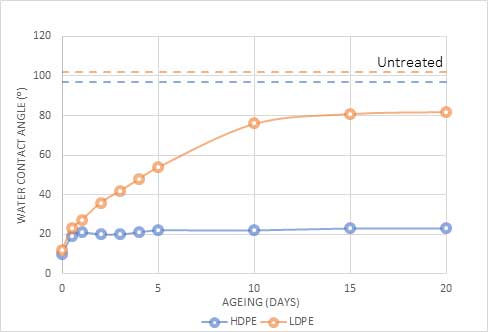
Fig 1: Change in water angle(⁰) of High-Density Polyethylene (HDPE) and Low-Density Polyethylene (LDPE) after plasma treatment.
There are many other intrinsic properties of a polymer that may have an effect on the rate of recovery, such as crystallinity, polarity, conductivity etc.
Figure 2 demonstrates the differences between three different polymers after undergoing the same plasma treatment and being stored under the same conditions. It can be seen that, not only is the rate of recovery different, but some polymers are treated much more effectively than others, with polystyrene (PS) remaining in a high state of activation even after several weeks.
Once again, it can also be seen that all of the materials remain in an activated state and never fully recover to the untreated low energy state level.
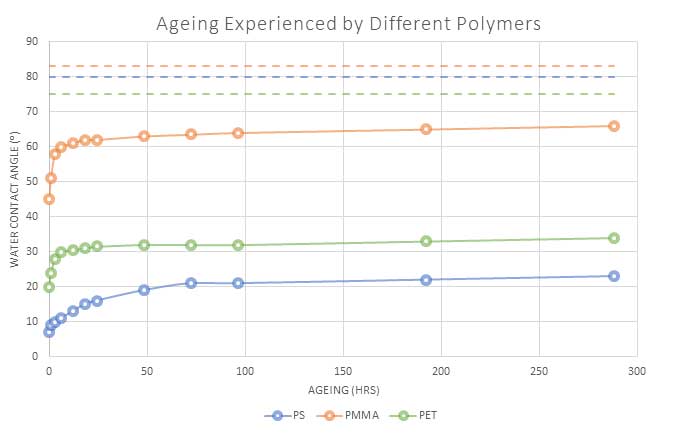
Fig 2: Water angle(⁰) of 3 different polymers after undergoing the same plasma treatment.
Duration of plasma treatment
There is some evidence that a longer exposure time to plasma may result in a higher number of surface and subsurface bonds of the material being broken by UV radiation from the plasma, leading to easier chain motion of molecules in the bulk of the material and hence a more rapid recovery process. Some research even states that plasma times of just 5 seconds are sufficient. Such short times however are not always enough for the first step removal of contamination and it’s therefore important to strike a balance.
Figure 3 shows the surface energy recovery behaviour of plasma treated PET for different plasma treatment times. Although the rate of recovery is faster for longer plasma treatment, this is still the most effective treatment time overall and results in a lower angle (higher surface energy) overall.
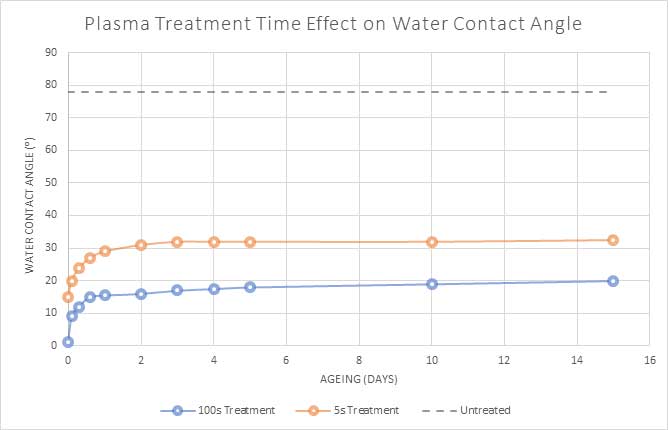
Figure 3: Plasma treatment of PET polymer for 5 seconds and 100 seconds
Storage Conditions
Storage conditions of plasma-treated materials can have a large effect on the energy state of the surface. Higher temperatures can lead to faster hydrophobic recovery due to the increased ease of mobility of chain structures beneath the surface for example.
Figure 4 shows the effect of storage temperature on a water angle of plasma-treated PET. It can be seen that higher temperature storage conditions contribute to the rapid recovery of the sample. In this example, water angle results show that the surface energy returns to almost 90% of its previous value in less than one hour.
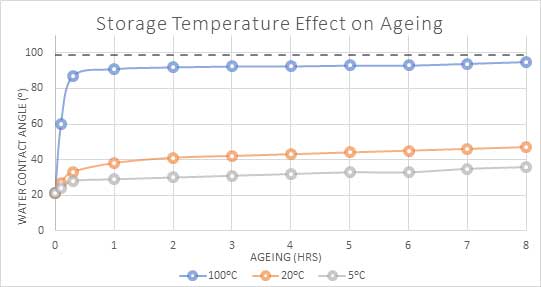
Fig 4: Water angle(⁰) of PET polymer over time when stored at different temperatures.
Airborne contamination and the transfer of oil and salts from with bare hands also have a detrimental effect on surface energy.
Covering the plasma-treated material will prevent recontamination but care must be taken that the covering itself does not transfer or leech contamination. Figure 5 compares two samples following plasma treatment; one that has been covered and one that has been left uncovered. The uncovered parts display an enormous variation in angle over time, probably due to different types and degrees of contamination on different parts of the surface, whereas the covered samples show a more stable expected recovery over time.
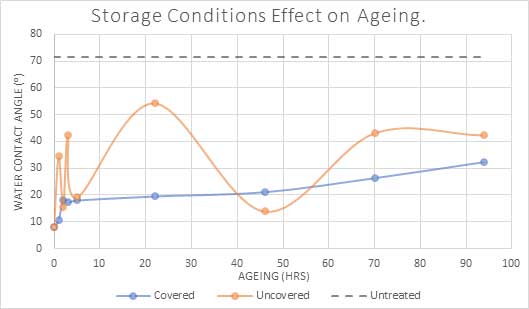
Fig 5: Water angle(⁰) of PET polymer over time when covered and handled with gloves, compared to uncovered and handled without gloves.
Type of plasma treatment
Another factor that can affect the rate of recovery of a plasma-treated material is the nature of the gas used in the plasma process. Different gases can introduce different functional groups onto the surface, which in turn leads to slightly different surface characteristics.
Figure 6 compares the measured water angle of plasma-treated PET for different process gases. The oxygen treated PET sample retained the lowest angle, although air showed the highest initial decrease. Air plasma processes are often perfectly suitable for treating materials due to the oxygen content within ambient air and also offer a cost-saving compared to using bottled gas.
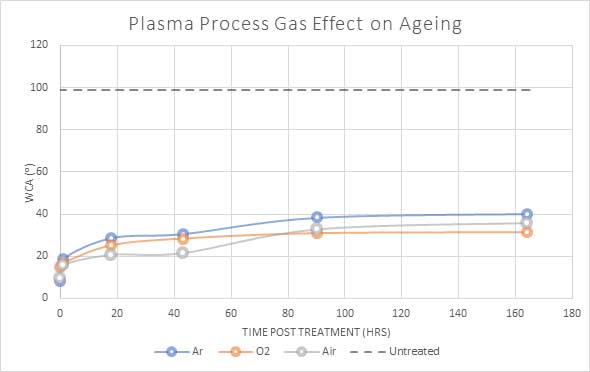
Fig 6: Water angle(⁰) of PET polymer when plasma treated with differing process gasses.
Example Water Angles
The following images show the change in water angle over time for a plasma-treated sample. Complete wetting of the sample can be seen immediately following plasma treatment, indicating high surface energy and excellent adhesion characteristics. The water angle increases over a 7-hr period as the sample slowly recovers, but always remains in a higher surface energy state than the untreated sample.
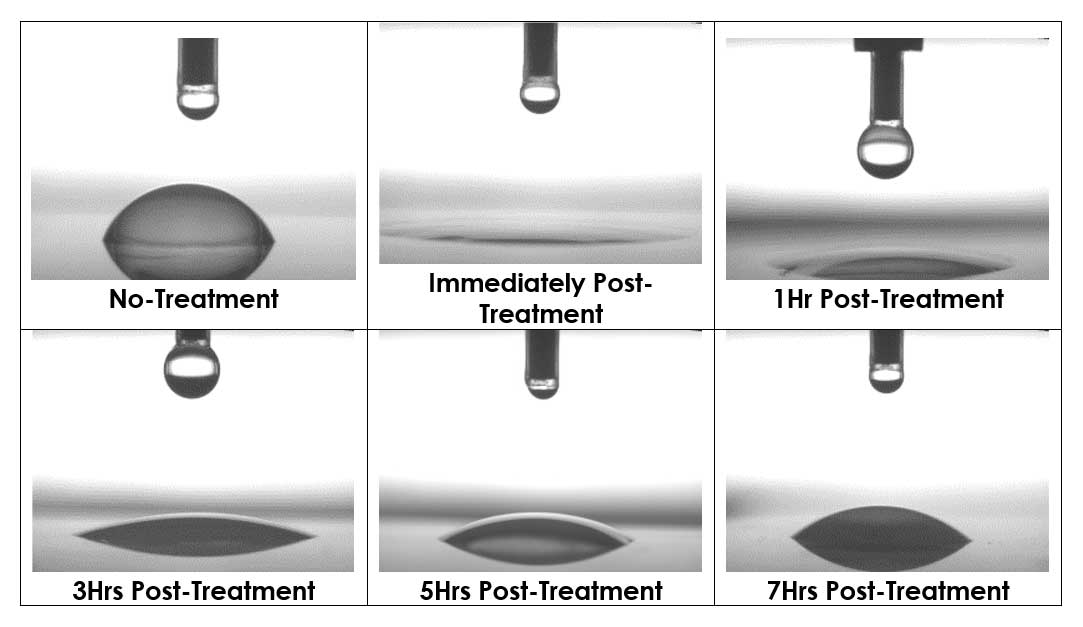
Conclusions
Each material must be considered individually when questioning how long a specific plasma treatment will last, although overall, most polymers will retain a higher surface energy than the untreated polymer for several weeks.
In general, for the best results, it is better to perform printing/bonding processes as soon as is practically possible following plasma treatment, however, if that isn’t possible then it is worth considering the following:
- Covering the parts if possible, using foil, not leaving the treated surface open to ambient air.
- Always using powder-free gloves to handle plasma-treated parts.
- Storing plasma-treated parts in as cool an area as possible, avoiding warm areas.
- Reducing long treatment times. Oxygen plasma is capable of fully cleaning and activating a polymer surface within a few minutes.
Henniker have an extensively equipped plasma processing and testing laboratory in our UK headquarters where we can conduct trials to determine both the effectiveness and lifetime of plasma treatment on a wide range of samples.
Please us today if you would like to learn more.
www.plasmatreatment.co.uk







